The BD Hypak™ for biotech is our versatile pre-fillable syringe delivery system that addresses key factors of compatibility with biopharmaceutical drugs.
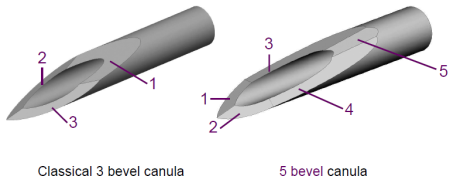
Hypak for Biotech – 5 Bevels Technology
Usually normal needles have 3 bevels at the end. Whereas, BD company has created 5 neat bevels by adding 2 new cuts at the end of the needle and has facilitated the penetration of needle into the skin.
This technique is patented by BD Corporation under the patent number US5752942
According to clinical studies, the use of 5bevel and 29G Thin Wall (TW) technologies in the needles increases 70% penetration and 40% reduction in pain caused by injection.
29G Thin Wall (TW) needle technology
BD has 20 years of experience in manufacturing thin-wall needles and meet ISO standards for cannula stiffness and resistance to breakage. As the gauge of the needle increases, its internal and external diameter decreases. Because of this, the patient’s pain will be reduced, but on the other hand, the flow of liquid in the needle will be reduced too. Reducing the flow will cause the plunger rod to retract and will make the injection difficult.
BD company has reduced the outer diameter of the needle to 29 gauge by changing the alloy of the needle, while its inner diameter remains the same as the inner diameter of the needle with 27 gauge. The new product is called 29Gauge Thin Wall and although it has a smaller diameter and less pain during injection, the flow of liquid has not decreased.