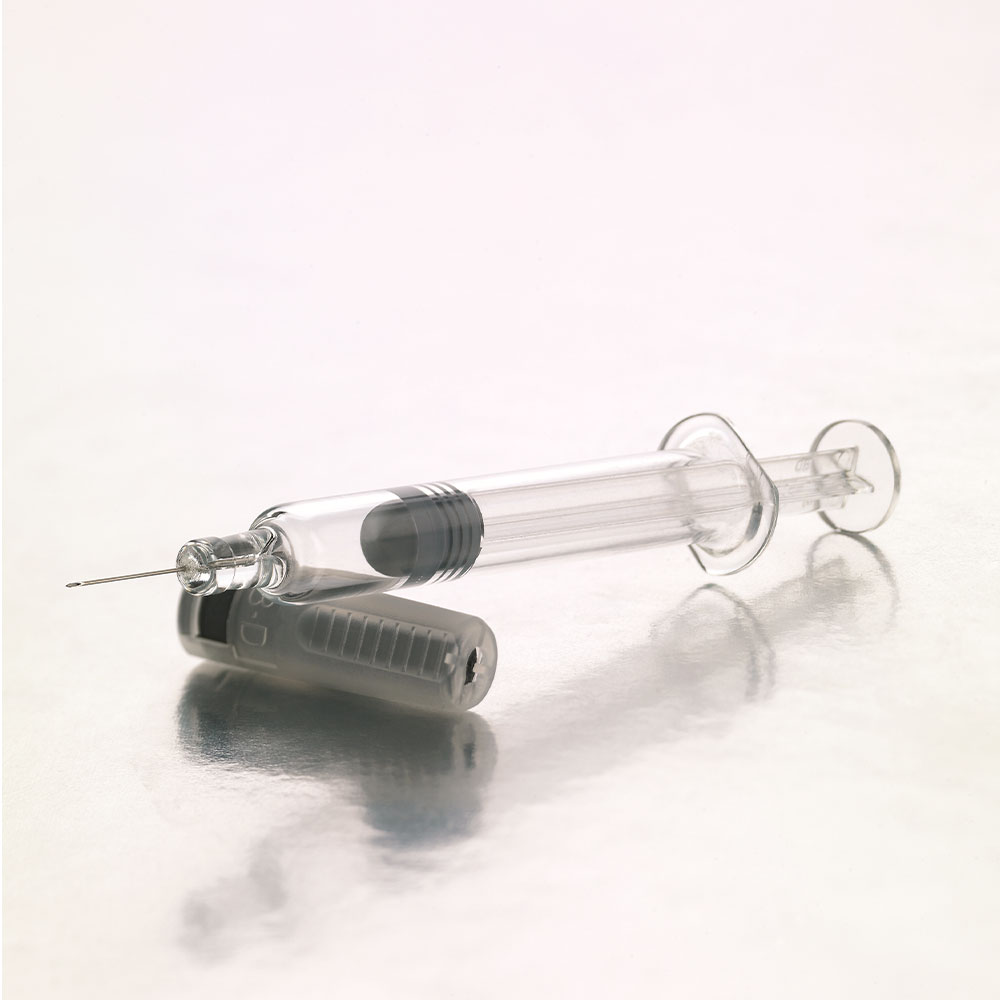
The BD Neopak™ syringe platform captures the essence of BD’s 30 year collaboration with biopharma industry, leveraged to best meet current and emerging requirements of sensitive biologic drugs for chronic disease patients. The BD Neopak™ platform has been developed with a goal to assure the most efficient combination of product development and commercialization.
The BD Neopak™ syringe is available in both 1-ml and 2.25 ml formats, and can be expanded to include BD Advanced Technologies such as BD XSi™, and BD VisioGuard™